At Apple’s “It’s Glowtime.” special event, the technology giant unveiled a pretty important and powerful health feature coming to AirPods Pro 2. Of course, that’s the forthcoming “Hearing Aid” functionality coupled with hearing protection and a hearing test feature – all of which would be arriving in a software update at no additional cost.
Now, three days after Apple unveiled the feature and noted that it was pending FDA authorization, a greenlight was just issued. The U.S. Food and Drug Administration (aka the FDA) authorized the AirPods Pro Hearing Aid feature as the “first over-the-counter (OTC) hearing aid software device.”
It’s a critical step for Apple, as they needed this approval to roll out the feature. Furthermore, it’s a big deal for the over-the-counter hearing aid industry, and Apple getting the first-ever authorization is a significant step.
The acting director of the FDA’s Center for Devices and Radiological Health said, “Today’s marketing authorization of an over-the-counter hearing aid software on a widely used consumer audio product is another step that advances the availability, accessibility and acceptability of hearing support for adults with perceived mild to moderate hearing loss.”
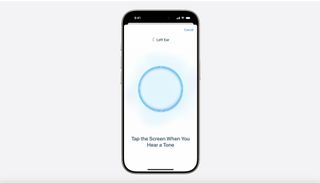
The FDA evaluated the feature “in a clinical study with 118 subjects with perceived mild to moderate hearing loss, at multiple U.S. sites,” and the results “demonstrated that subjects who used the HAF self-fitting strategy achieved similar perceived benefit as subjects who received professional fitting of the same device.” This validation by the FDA led to the overall marketing authorization of the feature for Apple, which likely matches the tests and studies that the technology company conducted internally.
AirPods Pro gaining the Hearing Aid feature is a big step, especially for folks with mild to moderate hearing loss. As an over-the-counter solution, and, actually, a software update that will hit existing AirPods Pro 2, it offers an alternative to purchasing other over-the-counter hearing aids, which can be more expensive.
Once available, to use the Hearing Aid feature on AirPods Pro 2, you’ll conduct the Hearing Aid test to establish a hearing profile, and then AirPods Pro 2 will make adjustments in real-time to boost sound. Apple notes that the AirPods Pro 2 will function as a “clinical-grade hearing aid,” and users will be able to make adjustments to tone, volume, and balance when they choose so.
Additionally, this authorization from the FDA allows Apple to roll out the hearing test feature on AirPods Pro 2, which TechRadar’s Editor-at-Large Lance Ulanoff had the chance to test. This feature, as well as hearing aid functionality and other hearing protections baked into AirPods Pro 2, move the earbuds past just being a device for listening and connecting with others. It builds upon noise level alerts for what you’re listening to on AirPods and environmental alerts for noise built into the Apple Watch.
With Apple gaining the FDA’s authorization for its AirPods Pro Hearing Aid feature, we’re likely one step closer to the feature being rolled out. As shared during the keynote and in a press release, Apple plans to release the Hearing Test and Hearing Aid features this fall in over 100 countries, including the United States, Germany, and Japan.
These will come in the form of a software update for AirPods Pro 2 in conjunction with an iPhone or iPad that is running iOS 18 or iPadOS 18.